StoryGraph Biggest Story 2021-04-24 -- fda cdc lift pause on j j (4), chief medical officer of johnson (4), vaccine cdc (3), the european medicines agency (3), said joanne waldstreicher (3)

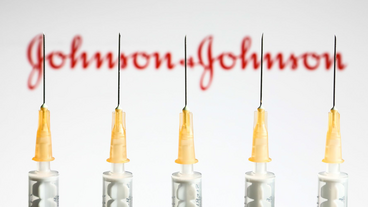
J&J Vaccine Reapproved After Review Of ‘Extremely Rare’ Blood Clots
A rare side effect, seen by 1.9 people per million doses given, forced the CDC and FDA to pause distribution of the COVID-19 vaccine last week.

Federal agencies lift pause on use of Johnson & Johnson vaccine, saying benefits outweigh risks
The U.S. decision to lift the pause on the vaccine's use with a warning added to the label is similar to one taken by Europe’s drug regulator.

FDA, CDC lift pause on J&J Covid vaccinations
After a panel recommended that the United States resume use of the Johnson & Johnson vaccine, the CDC officially lifted the pause.

Why the Johnson & Johnson vaccine pause was the right move
The CDC and FDA on Friday accepted recommendations that Johnson & Johnson Covid-19 vaccinations should resume, but with a warning.
This story was constructed with the SHARI Process:
- The StoryGraph Toolkit extracted URIs from the biggest story of the day from the StoryGraph service
- Hypercane performed the following steps:
- It accepted the list of original resource URIs from the output of the StoryGraph Toolkit, and queried the Memento Aggregator to find as many mementos as possible
- For resources that were not already mementos, it submitted them to web archives with ArchiveNow
- It analyzed all mementos to automatically discover the most frequent sumgrams and named entities present in the overall story
- It analyzed all images in these mementos to automatically select the best image for the overall story
- It then formatted the data for the story based on all of this input
- Raintale took the input from Hypercane and rendered the final product with information supplied by MementoEmbed
